India Pharma Outlook Team | Saturday, 28 June 2025
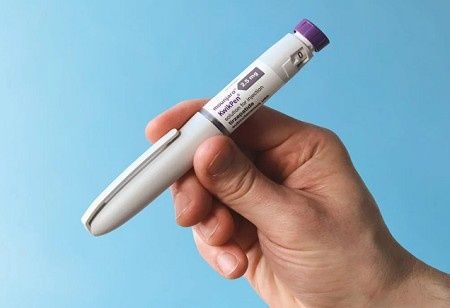
Eli Lilly has announced that its diabetes and obesity medicine, Mounjaro, will be available in a prefilled pen format in India, which can increase competition in the fast-growing chronic care space.
The company received approval from the Central Drugs Standard Control Organisation (CDSCO) to market the Mounjaro KwikPen in six strengths - 2.5?mg, 5?mg, 7.5?mg, 10?mg, 12.5?mg, and 15?mg - providing prescribers some discretion in how to adjust dosages for their patients.
The prefilled pen format follows a previous formulation that was in a vial, and should significantly impact patient compliance and convenience with once-weekly dosing.
This is important to note, as the launch comes at a time where India’s diabetes and obesity burden are increasing rapidly and is therefore a priority market for global pharma companies. Mounjaro − which has the active ingredient tirzepatide − belongs to the GLP-1 receptor agonist class that has proven it can help achieve blood glucose control and weightloss.
Also Read: Mass Spectrometry in Biomarker Discovery for Early Disease Detection
Eli Lilly's launch is especially noteworthy as it comes closely on the heels of Novo Nordisk’s Wegovy launch in India as a competing therapy. As the obesity and metabolic disorder treatment space heats up, the introduction of Mounjaro in a pen format is poised to deepen Eli Lilly’s presence in India’s high-growth lifestyle disease segment and reshape patient-centric care delivery.