India Pharma Outlook Team | Monday, 23 June 2025
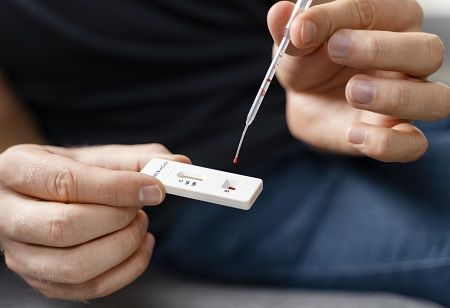
The Indian Council of Medical Research’s National Institute of Immunohaematology (ICMR-NIIH) has developed the world’s first simple point-of-care (POC) test for the rapid diagnosis of Haemophilia A and von Willebrand Disease (VWD).
This paper-based diagnostic test is expected to be used in primary healthcare facilities where the qualitative answer can be seen within less than 10 to 30 minutes with a small amount of plasma.
India has made a massive leap with the POC test since the country has approximately 1.4 lakh with Haemophilia and 1 crore may be VWD but have not been tested because testing facilities are not very accessible. The cost of this test is less such that at 200-250 compared to conventional diagnostics, which cost between 2,000 and 10,000. The sensitivity and specificity recorded by clinical trials are above 98% thus reliable and convenient to use even in rural settings.
Also Read: The Evolution of In-Vitro Diagnostics: Trends and Future Prospect
Its effectiveness in camps and outreach settings has then been shown in pilot programs, one in Kerala. The test will also hugely widen accession of early diagnosis and treatment to women with unexploited bleeding or with diseases such as menorrhagia and postpartum haemorrhage. ICMR is currently in search of industry to mass-produce and commercialize the test as it enters India at large into the field of medicine in an attempt to change the way bleeding disorders are diagnosed and handled.