India Pharma Outlook Team | Wednesday, 02 July 2025
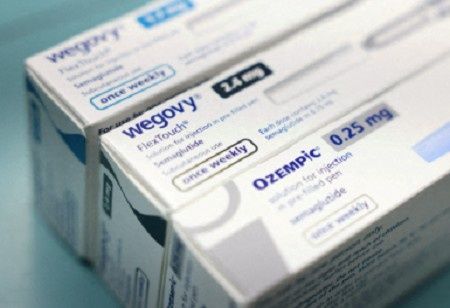
Biocon Ltd is seeking regulatory approval for its generic version of Ozempic (semaglutide) to get into Canada's high growth obesity drug market, aiming for a submission date of September 2025.
This would allow for a launch in early 2026 just after Novo Nordisk's patent expires in Canada. Biocon will also be looking to launch generic Wegovy, another biotech blockbuster in semaglutide, as part of a larger pipeline for peptides.
The aggressive push comes as demand for GLP-1 receptor agonists, which have increased demand globally, for both weight-loss and diabetes products. Biocon wants to be the first in this lucrative market, projected to be $100 billion worldwide by 2030, with semaglutide generating more than $25 billion alone with sales for 2024. Biocon expects to launch the product with a lower price point, but expects to offer only moderate pricing of 20% off, unlike with small molecule generics typically priced at steep discounts.
Also Read: Mass Spectrometry in Biomarker Discovery for Early Disease Detection
Biocon has already had regulatory success, with UK approval granted for its liraglutide generic. This approval has allowed Biocon more credibility in its space, following approval it is tested in India and expanding in Latam with further aspirations in Asia-pac. Biocon is aggressively seeking to establish itself as an option in the space following patents expiration as a well-established competitor with the highly competitive space.