India Pharma Outlook Team | Wednesday, 23 July 2025
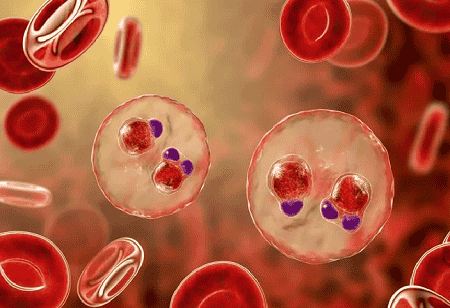
In a significant boost to the biopharma sector, the Indian Council of Medical Research (ICMR) has issued an Expressions of Interest (EoI) to license its indigenous malaria vaccine, AdFalciVax.
The vaccine targets Plasmodium falciparum, the deadliest species of malaria, and represents a significant advancement in India's journey to realization of made-in-India solutions for infectious diseases.
AdFalciVax is based on a recombinant, chimeric multi-stage vaccine that targets transmission and infection using the full-length PfCSP with a key part of PfsPro6C. The platform is based on Lactococcus plantaris and allows for scalability and efficiency.
ICMR is looking to form partnerships with organizations who are capable of the complete development, scale-up, manufacture and commercialize the vaccine. Organizations interested will need to provide proof of strong infrastructure and capacities, and demonstrate willingness to comply with ICMR's technical and regulatory requirements. Detailed knowledge access to technical dossiers, readiness for facility inspections, and independent financial commitment will need to be established.
Also Read: India Developing Multi-Stage Malaria Vaccine Named 'AdFalciVax'
ICMR will provide technical know-how, expert advice, and access to clinical infrastructure to facilitate vaccine development, but will not provide financial support, unless that is agreed to formally.The selected partner will take the lead in navigating regulatory approvals and large-scale production.
The deadline to submit EoIs is August 17, 2025, via the Medical Innovation Patent Mitra portal on ICMR’s website. This is a strategic opportunity for vaccine developers and biotech firms looking to advance public health while expanding innovation portfolios.